DIGE guide
Technique
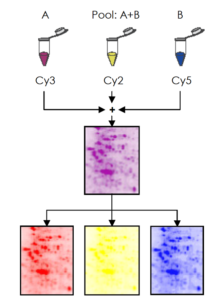 DIGE – Differential Gel Electrophoresis – is a variant of gel electrophoresis that allows for simultaneous separation of up to three samples on one gel, bringing a new level of statistical confidence and reliability to 2D gel electrophoresis.
DIGE – Differential Gel Electrophoresis – is a variant of gel electrophoresis that allows for simultaneous separation of up to three samples on one gel, bringing a new level of statistical confidence and reliability to 2D gel electrophoresis.
The system uses fluorescent cyanine dyes covalently bound to the proteins prior the 2D gel electrophoresis process. The dyes (CyTM2, Cy3 and Cy5) are mass and charge-matched, but have unique fluorescent properties. Therefore the labeled proteins migrate simultaneously on the 2-DE gel but still produce distinct excitation and emission spectra.
This means multiple protein samples labeled with different dyes can be co-migrated on the same gel. The gel is scanned using an adapted fluorescence scanner or camera system to generate overlaid, multi-channel images for each gel.
There are two different CyDyeTM DIGE Fluor dyes available.
- The minimal dyes are NHS ester dyes that label lysine residues, and are recommended for normal applications. Multiplexing up to three samples and labeling of 50 μg protein is possible.
- The saturation dyes are maleimide dyes that label cysteine residues, and should be used when samples are precious or available in very small amounts. Multiplexing up to two samples and labeling of 5 μg protein is possible.
Internal standard
This type of methodology allows the inclusion of an internal standard that ideally is a pool of all the samples within the experiment. The internal standard is labeled with one of the cyanine dyes (typically Cy2 for minimal dyes and Cy3 for saturation dyes) and run on each gel in the experiment. This creates an image that is the average of all experimental samples, with all proteins in the experiment represented. The presence of the internal standard in every DIGE gel provides an inherent link between samples that can be exploited to match and normalize the protein quantities across samples.
References
Unlü M., Morgan M.E., Minden, J.S. Difference gel electrophoresis: a single gel method for detecting changes in protein extracts. Electrophoresis. 1997 Oct;18(11):2071-7
Alban, A., David, S.O., Bjorkesten, L. et al. A novel experimental design for comparative two-dimensional gel analysis: Two-dimensional difference gel electrophoresis incorporating a pooled internal standard. Proteomics. 2003 Jan;3(1):36-44.
Advantages
The advantages of the DIGE technology using an internal standard are:
- Matching between gels is more straightforward. Matching between images of the same gel is implicit because running differences are eliminated for the corresponding samples. Matching between gels can be performed between internal standard images, for which variations in spot patterns are due only to electrophoretic differences.
- The use of the internal standard virtually eliminates experimental gel-to-gel variation and enables accurate quantification of induced biological change between samples. Quantitative comparisons of protein between samples are made based on the relative change of each protein spot to its own in-gel internal standard.
- No technical replicates need to be run to confirm differences in protein abundance, thus reducing the number of gels required per experiment as well as analysis time and cost.
Analysis of DIGE experiments in MelanieTM
The DIGE version of MelanieTM integrates functionality that fully exploits the advantages of DIGE (and other multiplex gel electrophoresis techniques):
- When setting up the project, you can specify if the experiment was run with an internal standard, and if so, on which dye.
- When importing DIGE images, it detects probable combinations of co-run images that can be validated with a single click. Importing from *.DS files allows fully automatic gel naming and grouping.
- Image editing operations such as flipping, rotating, scaling and cropping are carried out simultaneously on all images of the same gel.
- The experimental design setup works as with any other project. Note that the internal standard images should not be included in the design. They will be used automatically in alignment and spot normalization, but are not of interest to answer your study questions.
- The alignment strategy is automatically set up to implicitly match images within gels and use the internal standard images for matching between gels. However, the possibility exists to match within-gel images explicitly.
- By default, spot detection uses all sample and internal standard images in the experiment to generate a representative spot pattern. You can however choose to only utilize the internal standard images, to minimize nonsensical volume ratios. Indeed, spots present in an image but absent in the corresponding internal standard (where it will have a spot value close to zero) will anyway be quantitatively doubtful.
- Normalized Volume Ratios (ratio of normalized sample volume to internal standard volume) allow the comparison of relative spot quantities throughout the experiment. Read more about normalization.
- The tables, graphs and statistical analyses are adapted to the presentation of results in terms of Volume Ratios (in addition to the other quantification values available in the Classic version).
