Image alignment demystified: Part 1 – Understanding the fundamentals
Welcome to the first part of our blog series on aligning 2D gel and blot electrophoresis images using Melanie software. Our goal is to demystify the process of image alignment and offer guidance on achieving accurate results. Throughout this series, we’ll focus on key takeaways instead of providing detailed descriptions of software steps and features, which can be found in the Expression Analysis and Coverage Analysis user guides.
In this opening article, we’ll look at the core concepts and principles of image alignment.
Definitions and terminology
2D gel electrophoresis offers an unparalleled ability to separate and visualize thousands of proteins simultaneously. However, it encounters limitations such as positional variation, which can influence the uniform localization of protein spots across various gels. This inconsistency in protein migration can arise from minor fluctuations in the pH gradient, inconsistent electric fields, temperature differences during the running process, discrepancies in gel composition or thickness, and slight inconsistencies in sample loading or handling.
The problem of positional variation poses a challenge to coherent analysis of spots across different gel and blot images. Alignment comes into play here, providing a solution by adapting an image to the coordinate space of a reference image. The aim is to ensure that spots on the aligned image are in the same positions as their counterparts on the reference image. By performing this alignment on all images in an experiment, spot locations can be quantified consistently across the entire dataset. This allows accurate statistical analysis that avoids the problem of missing values.
Some vendors oppose alignment and matching, saying that alignment is finding correspondences at the pixel-level while matching focuses only on spot-level correspondences. This distinction can be misleading. In software like Melanie, matching spots is an integral part of the alignment process, and pixel-level correspondences are established.
Why image alignment is so critical
It is important to understand that alignment is the cornerstone of your analysis. Think of alignment as the keystone of a bridge; if it’s secure, everything else stands firm. If it’s shaky, the entire structure can crumble. A poor alignment can lead to inadequate spot detection, compromising the integrity of your data from the get-go. A sloppy alignment also becomes a gateway to extensive manual editing and interpretative dilemmas, which can be a significant drain on your resources and time. Finally, a hasty or imprecise alignment directly affects the robustness of your quantification and statistical results.
The alignment phase therefore deserves the lion’s share of your attention and effort. It’s the step where your time investment yields the richest dividends. Get it right, and you’ll find that subsequent stages of the analysis unfold with gratifying ease, saving time, effort, and ensuring the integrity of your conclusions.
Principles and steps of the alignment process
Definition of the alignment strategy
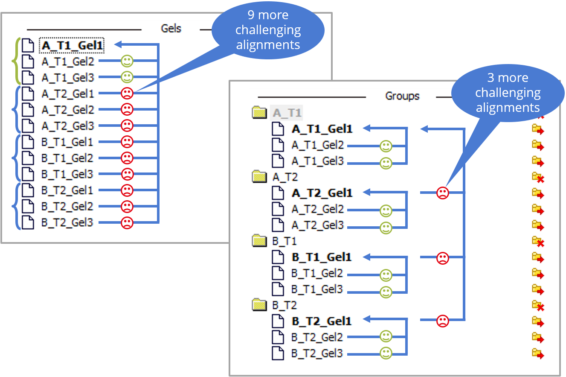
Aligning multiple images in an experiment requires a clever alignment strategy. This is especially true for conventional 2DE experiments. Indeed, not all images are equally easy to align. Sometimes, images belonging to the same treatment group are more similar, while other times spot patterns from gels run under identical experimental conditions align better, even if they represent different treatments.
In the figure below, we have gel images from four different treatment groups. Aligning all images against a common reference image (left) results in some images aligning easily, while most others pose challenges. To streamline and expedite the alignment process, Melanie allows you to specify your alignment strategy by grouping similar images in the Alignment setup. This approach reduces the number of difficult alignments (right).
Grouping images based on experimental factors or creating a custom group hierarchy allows you to tailor the alignment process to your experiment’s characteristics, reducing alignment effort significantly.
In practice, you align images within a group to a reference image selected for that group. At a higher level, different groups are aligned by aligning their reference images with the reference group’s image. The reference image of the top-level group serves as the global reference.
For multiplex and coverage projects, Melanie provides a default alignment strategy that automatically groups images from the same gel or blot. The alignment reference is set to either the internal standard image (DIGE) or the primary image (Coverage, Coverage DIGE/DIBE) within each group. In such cases you can proceed directly to the Alignment step unless you wish to change the reference group (gel or blot).
Please note that for expression analysis experiments, the Alignment setup appears as a separate step in the workflow. In Coverage experiments, the Alignment setup can be accessed as explained here.
Automatic alignment
Once you have finalised your alignment strategy and reached the Alignment step, Melanie starts the alignment process by automatically aligning the first image pair. Here’s how it works:
- The software performs a preliminary spot detection on both images of the pair—the reference image and the aligned image.
- Subsequently, the software attempts to identify corresponding spots through an iterative process called matching. After the establishment of a new spot correspondence—called a match—the algorithm performs an alignment and seeks a spot correspondence that matches this new alignment. The resulting automatic matches are visually represented by vectors linking the spots. The two small diamonds at the ends of each vector indicate the pixel positions being matched.
- The software then applies warping or deformation to the aligned image to minimize the match vectors. This transformation brings the spots from the aligned image into the coordinate space of the reference image. Note that the warped view of the aligned image is visible only when the Warp option is activated.
Alignment review
While the alignment algorithm aims for automatic matches, there may be missing or inaccurate matches. Melanie provides the ability to edit matches. However, before diving into extensive match editing, you should first align all image pairs, review the alignment results and possibly explore different alignment strategies. Thanks to the fast automatic alignment process, evaluating various alignment possibilities beforehand can save time and effort in the subsequent match editing phase.
In part 2 of this series, we’ll look specifically at metrics and tools for assessing alignment quality, identifying potential problems, and ensuring accurate spot correspondence.
At this stage you may be concerned about unmatched spots. Be reassured that proper alignment can be achieved with a relatively small number of matches, and not all spots need to be matched.
Importantly, the spot contours displayed on the aligned image are projections of the spots from the reference image, despite Melanie performing separate spot detections internally. This visual link greatly facilitates match inspection. It’s also worth mentioning that spot detection at this stage is only temporary, with final detection occurring later in the process, so there’s no need to worry about undetected or inaccurately split spots.
Match editing
You can edit matches to achieve better alignment results. Options include deleting automatic matches, creating new matches, and modifying existing ones. In part 3 of this series, we’ll provide essential recommendations for optimal image alignment, with a focus on match editing.
Edited or manually added matches are called user matches. Distinguishing between automatic and user matches is easy as they are visually distinct in both their selected and unselected states. Actions can be different for automatic and user matches. For example, when deleting matches, you can choose to delete only automatic matches or all matches. Similar options apply when aligning or unaligning images.
Depending on the view (Side by Side or Dual), there are slight differences in how matches are edited. However, both methods involve using the left and right mouse buttons while ensuring that the Align mode is selected in the Toolbar of the Display area.
After editing matches, systematically check your alignment using the Image overview. When you are satisfied, you can proceed to the next alignment using the Next/Back buttons or by selecting an image pair in the Tree view.
Conclusion
Congratulations! You’ve taken the first step towards becoming a Melanie image alignment expert. In this initial part of our blog series, we’ve covered the essential concepts and principles that underpin image alignment, emphasizing the importance of removing positional variation for precise analysis. You now understand the distinction between alignment and matching and are familiar with the key steps in the alignment process.
But we’re not stopping here! In the upcoming parts of this series, we’ll dive even deeper into the world of image alignment. We’ll explore how to evaluate alignment quality, share practical recommendations, and address vital considerations to ensure your image alignment is accurate and dependable.
So, stay tuned for Part 2, where we’ll guide you through effective alignment quality assessment and identification of alignment issues. With this knowledge, you’ll be well-equipped to elevate your gel electrophoresis experiments and achieve exceptional results. Happy aligning!